Flipping alkenes for more effective cancer drugs with fewer harmful side effects

Stephanie Baum
scientific editor

Robert Egan
associate editor

For the first time, chemists have discovered a unique way to control and modify a type of compound widely used in medicines, including a drug used to treat breast cancer.
The research, led by the University of Bristol and in the journal Nature, also found a new mechanism associated with the chemical reaction that enables the shape of the compound to be flipped from being right-handed to left-handed by simply adding a common agent to the chemical reaction.
Study lead author Varinder Aggarwal, professor of synthetic chemistry at the University of Bristol, said, "The findings change our understanding of the fundamental chemistry of this group of organic molecules. It presents exciting implications because the science allows us to make alternatives to the drug tamoxifen, with potentially greater potency and fewer unwanted side effects."
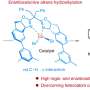
While most alkenes are easy to prepare, a specific type with four different parts鈥攃alled tetrasubstituted alkenes鈥攊s much more challenging, but used to make cancer-fighting medicines and natural products like essential oils.
So the research team aimed to find a more efficient method of making tetrasubstituted alkenes, including tamoxifen, which allows them to be easily manipulated and adapted into different forms.
The new method offers a highly versatile solution to building complex tetrasubstituted alkenes from simple building blocks.
Aggarwal explained, "Our original design plan used organic boronic esters as the key ingredient but that resulted in unstable intermediates, so it didn't work.
"We then tried a less common form of boron-containing molecules, namely boranes, and that's when the clever molecular gymnastics became possible. This new boron system enabled the installation of different groups on the alkene in a controlled manner from very simple building blocks, like Lego.
"It's so exciting because it holds the key to finding even better drug molecules鈥攍ike alternatives to tamoxifen鈥攚ith more of the properties you want and less of what is undesirable, such as side effects."
The scientists enlisted the help of computational chemists at Colorado State University to map exactly what was happening. That led to the full extent of their discovery being uncovered.
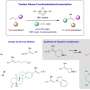
Co-author Robert Paton, professor of chemistry at Colorado State University, said, "The mechanism showed that by just changing the reaction conditions through adding an agent, the geometry of the alkene can switch direction from left to right. This was surprising and hadn't been seen before."
In addition to drug molecules like tamoxifen, the researchers also worked with natural products such as 纬-bisabolene, a fragrant compound found in essential oils, to demonstrate the broad applications of their breakthrough.
Aggarwal added, "Now that we have struck upon an effective, flexible methodology, it allows us to swap in other molecules, so the potential here is wide-reaching for both drug discovery and materials science."
More information: Varinder Aggarwal, Boron-mediated modular assembly of tetrasubstituted alkenes, Nature (2025). .
Journal information: Nature
Provided by University of Bristol